UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact Name of Registrant as Specified in its Charter)
|
|
|
|
|
||
|
|
|||||
(State or other jurisdiction of |
|
(Commission |
|
(I.R.S. Employer |
||
|
|
|
||||
|
||||||
(Address of principal executive offices) |
|
(Zip Code) |
||||
Registrant’s telephone number, including area code:
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class |
|
Trading |
|
Name of each exchange on which registered |
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 7.01 Regulation FD Disclosure
Prelude Therapeutics Incorporated (the "Company") has prepared investor presentation materials with information about the Company, which it intends to use as part of investor presentations. A copy of the investor presentation materials to be used by management for presentations is attached as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
The information in this Current Report on Form 8-K and in Exhibit 99.1 attached hereto is being furnished, but shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (“Exchange Act”), and is not incorporated by reference into any filing of the Company under the Securities Act of 1933, as amended, or the Exchange Act, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
|
|
|
Exhibit |
|
Description |
|
|
|
99.1 |
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL Document) |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
PRELUDE THERAPEUTICS INCORPORATED
|
|
|
|
|
Date: February 14, 2023 |
By: |
/s/ Laurent Chardonnet |
|
|
Laurent Chardonnet |
|
|
Chief Financial Officer |

SVB Global Biopharma Conference Presentation February 2023 Exhibit 99.1

Forward Looking Statements This presentation contains “forward-looking” statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to: our plans to develop and commercialize small molecule therapies, our expectations about timing and ability to commence, enroll or complete clinical studies, present data and clinical results or updates, and to obtain regulatory approvals for PRT543, PRT811, PRT1419, PRT2527, PRT3645, PRT3789 and other candidates in development, the ability of our product candidates to treat various cancers, the ability to discover additional suitable candidates for regulatory approval, the potential impact of the COVID-19 pandemic, and the sufficiency of our cash and cash equivalents to fund our operations. Any statements contained herein or provided orally that are not statements of historical fact may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by such terminology as ‘‘believe,’’ ‘‘may,’’ ‘‘will,’’ ‘‘potentially,’’ ‘‘estimate,’’ ‘‘continue,’’ ‘‘anticipate,’’ ‘‘intend,’’ ‘‘could,’’ ‘‘would,’’ ‘‘project,’’ ‘‘plan,’’ ‘‘expect’’ and similar expressions that convey uncertainty of future events or outcomes, although not all forward-looking statements contain these words. Statements, including forward-looking statements, speak only to the date they are provided (unless an earlier date is indicated). Certain data in this presentation are based on cross-study comparisons and are not based on any head-to-head clinical trials. Cross-study comparisons are inherently limited and may suggest misleading similarities or differences. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or other jurisdiction. These forward-looking statements are based on the beliefs of our management as well as assumptions made by and information currently available to us. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. If such assumptions do not fully materialize or prove incorrect, the events or circumstances referred to in the forward-looking statements may not occur. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations, except as required by law. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. Additional risks and uncertainties that could affect our business are included under the caption “Risk Factors” in our Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission for the three months ended September 30, 2022 and in our upcoming Annual Report on Form 10-K for the year ended December 31, 2022.

Prelude Therapeutics: Delivering Precision Medicines to Patients with Cancer Large Commercial Opportunities Diversified Pipeline Exceptional Team Well Capitalized Powerful R&D Engine
Prelude Discovery and Development Engine: Positioned to Succeed R&D ENGINE DIFFERENTIATED PIPELINE APPROACH CDK9 inhibitor MCL1 inhibitor SMARCA2 degrader CDK4/6 inhibitor Internal Discovery Engine Clinical Development Expertise Target Mechanisms that drive meaningful benefit in patients with cancer Discover Optimized molecules that overcome the limitations of current treatments Translate Science into meaningful treatments for patients

Differentiated Pipeline with Transformative Potential for Patients with Cancer Potentially Best-in-Class Selectivity Potential to avoid off-target toxicity and higher clinical activity Potent and Selective Degrader Potential to address major unmet need in biomarker-selected patients Optimized PK Profile Potential for maximal target engagement and improved cardiac safety Highly Selective Differentiated Metabolic Profile Potential for high tissue and brain penetration and better combinability CDK9 inhibitor MCL1 inhibitor SMARCA2 degrader CDK4/6 inhibitor

Former CMO Former CEO General Partner Experienced Management Team: Proven Track Records Board of Directors Victor Sandor, MD Paul Friedman, MD CEO David Bonita, MD Julian C. Baker Managing Member Baker Brothers Investments Kris Vaddi, PhD Founder & Chief Executive Officer Mardi Dier Former CFO, CBO Former CFO Andrew Combs, PhD Executive Vice President and Head of Chemistry Peggy Scherle, PhD Chief Scientific Officer Former CEO Martin Babler Jane Huang M.D. President and Chief Medical Officer Kris Vaddi, PhD Founder & Chief Executive Officer Laurent Chardonnet Chief Financial Officer

PROGRAM CANCER INDICATIONS DISCOVERY IND ENABLING PHASE 1 PHASE 2/3 AREAS OF CLINICAL FOCUS PRT2527 (CDK9 Inhibitor) Selected solid and hematologic malignancies R/R MCL, CLL, Aggressive Lymphomas as Monotherapy or in Combination with BTKi PRT1419 (MCL1 Inhibitor) Selected hematologic malignancies and solid tumors CLL Post Ven/BTKi, AML in combo with Azacitidine/Venetoclax PRT3645 (Next Generation CDK4/6 Inhibitor) Selected Solid tumors HR+ Breast cancer treatment through multiple lines, GBM, NSCLC in combination with kras inhibitors PRT3789 (SMARCA2 Degrader) Multiple genomically- selected cancers SMARCA4 deleted NSCLC and Other cancers New Programs (Multiple targets) Selected solid and hematologic malignancies Solid Tumors Heme Malignancies Prelude Precision Oncology Pipeline: Diversified and Differentiated

PRT2527 CDK9 Inhibitor

CDK9 Inhibition: Targeting Cancer by Regulating Oncogene Expression CDK9 regulates expression of several oncogenes that drive cancer cell growth and resistance (i.e. MYC, MYB, MCL1) Non-selective CDK9 inhibitors have demonstrated clinical activity in multiple tumor types but poor tolerability Improving the selectivity of CDK9 inhibitors may translate to better activity and safety SUPER ENHANCER RNA Pol II TSS MYC & MYB TARGET GENES mRNA

PRT2527: Potent and Highly Selective CDK9 Inhibitor Compound AZD4573 KB0742 VIP152** PRT2527 Biochemical* IC50 (nM) CDK9 1.9 483 16 0.95 Proliferation* IC50 (nM) 11 915 84 18 Plasma* IC50 (nM) 192 1056 923 196 Fold Selectivity CDK9 vs Other Isoforms CDK1 23x >20x 371x 73x CDK2 35x >20x 147x 340x CDK3 2x >20x 37x 35x CDK4 53x >20x 38x 250x CDK5 37x >20x >600x >1000x CDK6 79x >20x 296x >1000x CDK7 150x >20x >600x >1000x Highly Selective, ATP Competitive CDK9 Inhibitor >100x 100-10x <10x *Internal data; biochemical assay at 1 mM ATP, H929 CTG proliferation assay; **VIP151 was formerly BAY151 and licensed to Vincerx by Bayer

CDK9 inhibitor: PRT2527 Phase 1 Dose-Escalation Study in Advanced Solid Tumors Phase 1 dose escalation study of PRT2527 is ongoing and enrolling following tumor types Selected sarcomas displaying a gene fusion Castrate resistant prostate cancer HR+ HER2- breast cancer Non-small cell lung cancer Solid tumors with MYC amplification Nine patients have been treated in the first three dose levels (3, 6 and 12 mg/m2 I.V. weekly), with no dose-limiting toxicities and acceptable tolerability to date Dose-dependent inhibition of CDK9 transcription targets observed in PBMCs HR+ Hormone receptor positive; HER2- Human epidermal growth factor negative ClinicalTrials.gov Identifier: NCT05159518 ASH Annual Meeting 2022 Abstract No. 210

CDK9 Inhibitor: PRT2527 Phase 1 Studies in Solid Tumors and Hematologic Malignancies PRT2527 Solid Tumors N=11 PRT2527 MYC Amplified or Overexpressed Solid Tumors, Prostate Cancer N=15 Solid Tumors Dose dependent increases in exposure and target engagement observed in Phase 1 Clinical MYC and MCL1 depletion to levels consistent with tumor regression in preclinical models Generally well tolerated Dose Confirmation Dose Escalation PRT2527 Monotherapy Aggressive B cell lymphomas (multiple types), follicular lymphoma, CLL/SLL/Richters, MCL PRT2527 N=30 Hematologic Malignancies ASH 2022 preclinical oral presentation CDK9 as a target externally validated in aggressive lymphoma and other heme malignancies Dose Confirmation Dose Escalation ClinicalTrials.gov Identifier: NCT05159518 Solid Tumor data in 1H 2023 RP2D in hematological malignancies 2H 2023 Initial clinical data in 2H 2023

CDK9 Inhibitor Differentiation and Market Opportunity Potential for Improved Safety Based on Best-in-Class Kinome Selectivity PRT2527 is a highly potent CDK9 inhibitor with best-in-class kinome selectivity compared to competitor compounds Optimized PK profile to maximize therapeutic window Well-tolerated in GLP preclinical studies at doses exceeding those required for efficacy High levels of inhibition of CDK9 dependent genes in Phase 1 Market Opportunity CDK9 inhibitors in CLL, Mantle cell lymphoma, and DLBCL may address areas of high unmet need There are ~ 50,000 DLBCL patients , 55,000 CLL patients, and 25,000 mantle cell patients treated each year in the US CDK9 inhibitor MCL1 SMARCA2 CDK4/6

PRT1419 MCL1 Inhibitor

MCL1 inhibition: Targeting Cancer Cell Survival MCL1 is a member of the BCL2 family of inhibitors of apoptosis Established resistance mechanism to the BCL2 inhibitor Venetoclax Prolonged depletion of MCL1 is undesirable and may be associated with cardiac toxicity Optimizing the PK profile of an MCL1 inhibitor may maximize the therapeutic window Mechanism

MCL1 inhibitor: PRT1419 Phase 1 Study in Hematologic Malignancies PRT1419 Monotherapy AML/MDS/CMML CLL/SLL FL/MZL/MCL N=24-30 PRT1419 Combination PRT1419+Aza: AML/MDS/CMML PRT1419+Ven: AML/MDS/CMML PRT1419+Ven: MCL N=24-30 In the solid tumor PRT1419 dose escalation Phase 1, 26 patients have been treated and 15 patients @ RP2D No cardiac toxicity seen @ RP2D as measured by ejection fraction decline/troponin elevation Solid tumor data to be presented 1H 2023 Upregulation of MCL1 is a mechanism of resistance to BCL2 inhibition, particularly in CLL and AML; Strong preclinical hypothesis in heme1 Dose Confirmation Dose Escalation ClinicalTrials.gov Identifier: NCT05107856 RP2D in heme monotherapy expected 2H 2023 Initial clinical data in 2H 2023 1 Ong et al. Cancer Drug Resist 2022;5:380-400

MCL1 Inhibitor Differentiation and Market Opportunity Optimized PK Profile to Achieve Desired Target Engagement MCL1 Inhibitor CDK9 SMARCA2 CDK4/6 PRT1419 is a highly potent and selective MCL1 inhibitor Designed to have a PK profile with high clearance to provide desired target engagement with improved safety No cardiotoxicity or troponin changes in GLP preclinical studies at doses exceeding those required for efficacy No evidence of cardiotoxicity in the solid tumor Phase 1 at the recommended Phase 2 dose Market Opportunity AML, MDS, CLL, MCL patients need additional treatment options There are ~ 37,000 AML patients , 55,000 CLL patients, and 25,000 mantle cell lymphoma patients treated each year in the U.S.

PRT3645 CDK4/6 Inhibitor

CDK4/6 Inhibition: Targeting Cancer Through Cell Cycle Regulation Mechanism Validated mechanism with approval of CDK4/6 inhibitors in HR+ breast cancer Resistance mechanism to other targeted therapies including KRAS G12C inhibitors Current CDK4/6 inhibitors limited by poor tolerability and lack broad tissue penetration Next generation CDK 4/6 inhibitor with improved tolerability and tissue penetrance could translate into activity in areas of unmet need beyond HR+ breast cancer Sequential use of CDK 4/6 inhibitors in breast cancer may also improve outcomes

PRT3645 – Highly Selective CDK4-Biased Next Generation CDK4/6 Inhibitor Compound Palbociclib Abemaciclib PRT3645 Biochemical* IC50 (nM) CDK4 25 5 3 Proliferation* IC50 (nM) 52 70 47 Phospho-Rb* IC50 (nM) 28 30 16 Fold Selectivity CDK4 vs Other Isoforms CDK6 1x 6x 5x CDK1 >500x >500x >500x CDK2 >500x 173x >500x CDK3 >500x 212x >500x CDK5 >500x >500x >500x CDK7 >500x >500x >500x CDK9 209x 59x >500x >500x 500-50x 50-5x <2x *Internal data; biochemical assay at 1 mM ATP, MCF7 CTG proliferation assay; MCF7 pRB Highly Selective, ATP Competitive
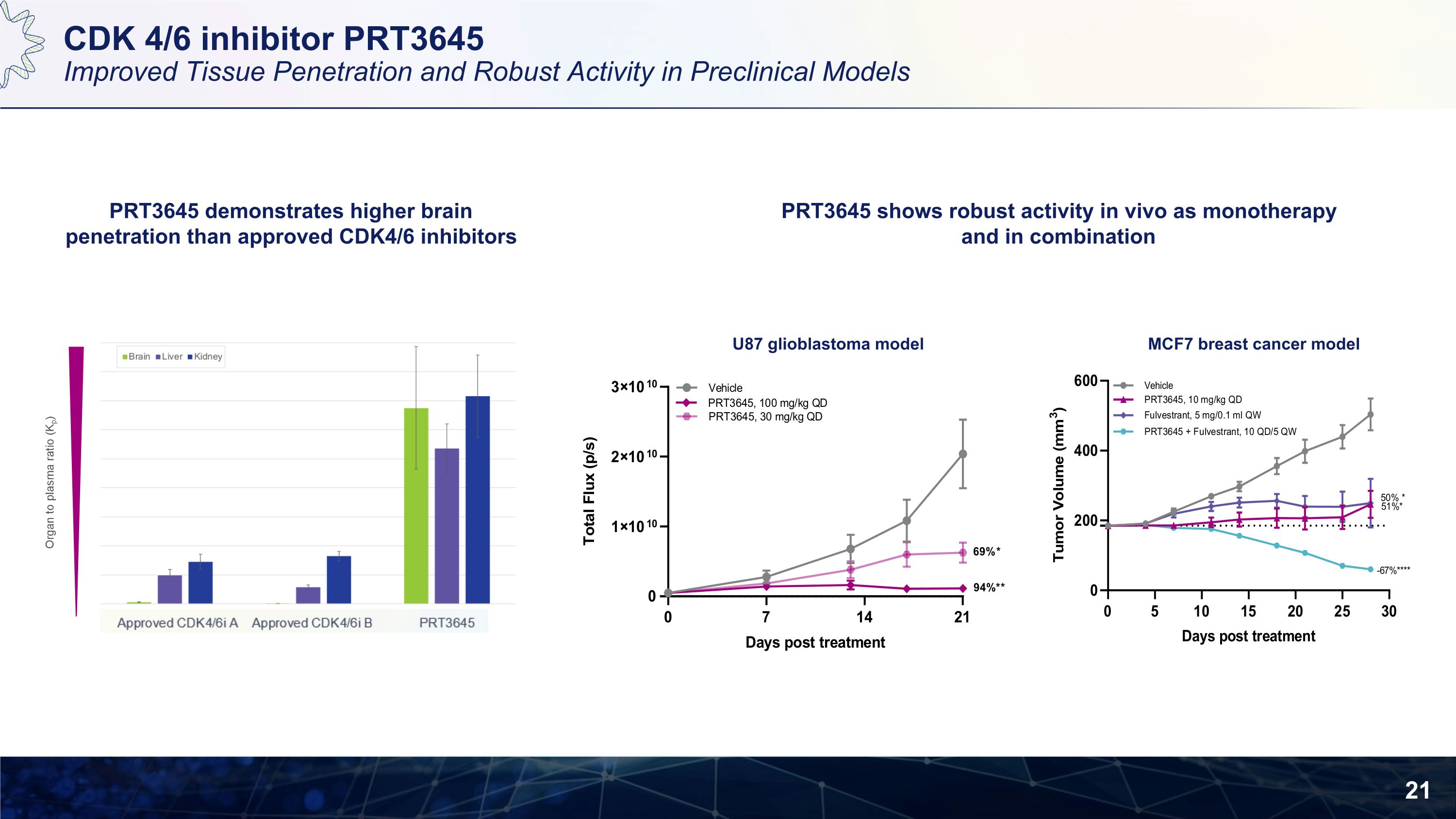
CDK 4/6 inhibitor PRT3645 Improved Tissue Penetration and Robust Activity in Preclinical Models PRT3645 demonstrates higher brain penetration than approved CDK4/6 inhibitors PRT3645 shows robust activity in vivo as monotherapy and in combination U87 glioblastoma model MCF7 breast cancer model

CDK4/6 Inhibitor: PRT3645 Phase 1 Study in Solid Tumors A differentiated and highly brain penetrant CDK4/6 inhibitor Potential to extend the reach of CDK4/6 inhibition beyond HR+ breast cancers, for which the first generation CDK4/6 inhibitors were approved PRT3645 Biomarker enriched patients with select tumor types including sarcomas, mesothelioma, gliomas, head and neck cancers and non-small cell lung cancer, in addition to breast cancer with or without brain metastases Dose Escalation and Confirmation ClinicalTrials.gov Identifier: NCT05538572 Initial clinical data in 2H 2023 RP2D in solid tumors in 2H 2024

CDK4/6 Inhibitor Differentiation and Market Opportunity Deep Tissue Penetration with Potential for Activity in Areas of Unmet Need PRT3645 is a highly potent and selective CDK4/6 inhibitor Optimized to demonstrate deep tissue penetration including brain penetrance Improved metabolism profile to allow for combination treatment in diseases beyond breast cancer Reduced toxicity in preclinical GLP studies with potential for improved tolerability in the clinic Market Opportunity: Breast cancer patients may benefit from sequential CDK 4/6 inhibitors treatment There are estimated to be 65,000 breast cancer patients treated with CDK 4/6 inhibitors in 2023 in the U.S. Other solid tumors (lung cancer, glioma, HER2+ breast cancer) may demonstrate activity in combination MCL1 CDK9 SMARCA2 CDK4/6 Inhibitor

PRT3789 SMARCA2 Degrader

Targeting SMARCA2 (BRM): Leveraging Synthetic Lethality The chromatin remodeling (SWI/SNF) complex is frequently mutated in cancer making it a potential therapeutic target Activity of the SWI/SNF complex requires either SMARCA4 (BRG1) or SMARCA2 (BRM) Loss of SMARCA4 (BRG1) through mutation leads to dependency on SMARCA2 (BRM) Subsets of solid tumors express SMARCA4 (BRG1) mutations Selectively inhibiting SMARCA2 (BRM) offers an attractive approach to target SMARCA4 (BRG1) mutant tumors Mechanism

Indication Any SMARCA4 Mutation1 NSCLC 10.0% Esophageal 8.0% Gastric (stomach adeno) 8.3% Skin (invasive and in situ melanoma)* 21.0% Endometrial (uterine corpus) 13.3% Squamous cell lung 7.7% Urinary (bladder) 9.0% Colorectal 6.0% Pancreatic 2.9% Melanoma (invasive) 8.7% SMARCA4 Mutations in NSCLC: An Opportunity with No Approved Therapies SMARCA4 Prevalence across selected Solid Tumors 1.cBioPortal; FoundationCore; 2.SMARCA4 LOF mutations included homozygous missense, hotspot mutations with LOF, and damaging mutations; 3.SEER 2022; Globocan; * Source: American Cancer Society – Cancer Facts & Figures 2022 Fernando et al. Nature Communications 2020 SMARCA4 Deletion – A Novel Biomarker for NSCLC Targets for Approved Drugs

PRT3789: Potent and Selective SMARCA2 Degrader with In Vivo Activity Robust Tumor Growth Inhibition of SMARCA4 mutated but not WT Xenograft SMARCA4 mutant SMARCA4 WT PRT3789 PRT3789 Vehicle Low Dose Mid Dose High Dose SMARCA2 SMARCA4 LAMIN B1 SMARCA2 SMARCA4 LAMIN B1 Male Female Significant Degradation of SMARCA2 Protein but not SMARCA4 in Preclinical Models

SMARCA2 Degrader: PRT3789 Phase 1 Study in Solid Tumors SMARCA2 inhibition has the greatest potential in patients with SMARCA4 deficient cancers, including approximately 5-10% of all non-small cell lung cancers SMARCA2 degradation to be evaluated in Phase 1 Study population: advanced, recurrent, or metastatic disease, with loss of SMARCA4 due to truncating mutation and/or deletion Biomarker selected by local NGS or IHC in tumor tissue or blood HR+ and HER2-negative or HR+ and HER2+breast cancer Recurrent GBM (IDH wild type) or CDKN2A/B homozygous deleted IDH-mutant astrocytoma KRAS-mutant NSCLC CDK pathway alternation in any of the following tumor types: malignant mesothelioma, HPV-negative HNSCC (including oral cavity, oropharynx, hypopharynx, and larynx), sarcoma, or NSCLC ClinicalTrials.gov Identifier: NCT05538572 PRT3789 Solid Tumors with loss of SMARCA4 Backfill: up to 10 participants with a minimum of 6 NSCLC participants with loss of SMARCA4 Dose Escalation and Confirmation IND cleared Q4 2022 Provide Clinical update 2H 2023

SMARCA2 Differentiation and Market Opportunity Potential First-in-Class SMARCA2 (BRM) Targeted Protein Degrader PRT3789 is a potent and highly selective first-in-class SMARCA2 Degrader Designed to achieve the requisite high selectivity for SMARCA2 over the related isoform, SMARCA4, through a targeted protein degrader approach Improved tolerability compared to non-selective SMARCA2 inhibition Robust efficacy in SMARCA4 mutant preclinical models, providing clear patient selection strategy in the clinic Market Opportunity: 70,000 patients with SMARCA4 mutation in the US/EU5 SMARCA2 Degrader

Driving The Programs to Key Milestones and Value Creation PRT2527 PRT1419 PRT3645 SMARCA2 CDK9 MCL1 CDK4/6 Present solid tumor data in 1H RP2D in solid tumors in early-2023 RP2D in hematological malignancies in 2H Present initial clinical data for hematological malignancies in 2H Present solid tumor data in 1H RP2D in hematological malignancies in 2H Present initial clinical data for hematological malignancies in 2H Present initial clinical data in 2H PROGRAM 2023 MILESTONES Initiate Phase 1 in 1Q Provide Clinical update 2H PRT3789

Prelude Therapeutics: Key Takeaways and Reasons to Invest Confidential Current cash runway expected through Q4 2024 Potentially first-in-class SMARCA2 degrader program with a significant lead over competitors and offers transformational potential for the company Opportunity to drive programs to key inflection points in the next 12 – 24 months Emerging clinical data on CDK9 and MCL-1 programs demonstrate the potential for class-leading opportunities -CDK9 as a target externally validated in DLBCL with significant clinical and commercial potential Deep clinical pipeline with unique and potentially best-in-class or first-in-class molecules
