UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
(Address of principal executive offices, including zip code)
Registrant’s telephone number, including area code:
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| Item 7.01. | Regulation FD Disclosure |
Prelude Therapeutics Incorporated (the “Company”) has prepared investor presentation materials with information about the Company, which it intends to use as part of investor presentations. A copy of the investor presentation materials to be used by management for presentations is attached as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
The information furnished with this report, including Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any other filing under the Exchange Act or the Securities Act of 1933, as amended, except as expressly set forth by specific reference in such a filing.
| Item 9.01 | Financial Statements and Exhibits |
(d) Exhibits
| Exhibit No. | Description | |
| 99.1 | Presentation | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL Document) | |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| PRELUDE THERAPEUTICS INCORPORATED | ||||||||
| Date: January 10, 2022 | By: | /s/ Laurent Chardonnet | ||||||
| Laurent Chardonnet | ||||||||
| Chief Financial Officer | ||||||||

Precision Oncology Redefined Corporate Presentation January 2022 Exhibit 99.1

Forward Looking Statements This presentation contains “forward-looking” statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to: our plans to develop and commercialize small molecule therapies, our expectations about timing and ability to commence, enroll or complete clinical studies and to obtain regulatory approvals for PRT543, PRT811, PRT1419, PRT2527 and other candidates in development, the ability of our product candidates to treat various cancers, the ability to discover additional suitable candidates for regulatory approval, the potential impact of the COVID-19 pandemic and the sufficiency of our cash and cash equivalents to fund our operations. Any statements contained herein or provided orally that are not statements of historical fact may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by such terminology as ‘‘believe,’’ ‘‘may,’’ ‘‘will,’’ ‘‘potentially,’’ ‘‘estimate,’’ ‘‘continue,’’ ‘‘anticipate,’’ ‘‘intend,’’ ‘‘could,’’ ‘‘would,’’ ‘‘project,’’ ‘‘plan,’’ ‘‘expect’’ and similar expressions that convey uncertainty of future events or outcomes, although not all forward-looking statements contain these words. Statements, including forward-looking statements, speak only to the date they are provided (unless an earlier date is indicated). These forward-looking statements are based on the beliefs of our management as well as assumptions made by and information currently available to us. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. If such assumptions do not fully materialize or prove incorrect, the events or circumstances referred to in the forward-looking statements may not occur. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations, except as required by law. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. Additional risks and uncertainties that could affect our business are included under the caption “Risk Factors” in our Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission for the three months ended September 30, 2021.

Prelude Therapeutics: Vision Build a fully integrated oncology company on the foundation of drug discovery excellence to deliver novel precision oncology medicines to patients with underserved cancers

Powered by experienced drug developers with a proven track record of multiple successful oncology medicines Internal Discovery Engine Prelude Therapeutics: Building for Success Effective integration between cancer biology and medicinal chemistry to rapidly iterate and discover optimized molecules in a target class agnostic fashion Differentiated R&D Approach Clinical trial designs in selected cancer patients allowing efficient ‘go / no go’ decisions in caner types with potential for rapid regulatory approval Focused Clinical Development in Underserved Cancers Strong Financial Position: ~$320M Cash & Marketable Securities (9/30/21) 4 INDs approved in 4 years; 4 differentiated clinical candidates currently advancing through Phase 1 and into Phase 2/3 clinical development; 2 new additional INDs expected in 2022 Strong Execution and Commitment to Discovery
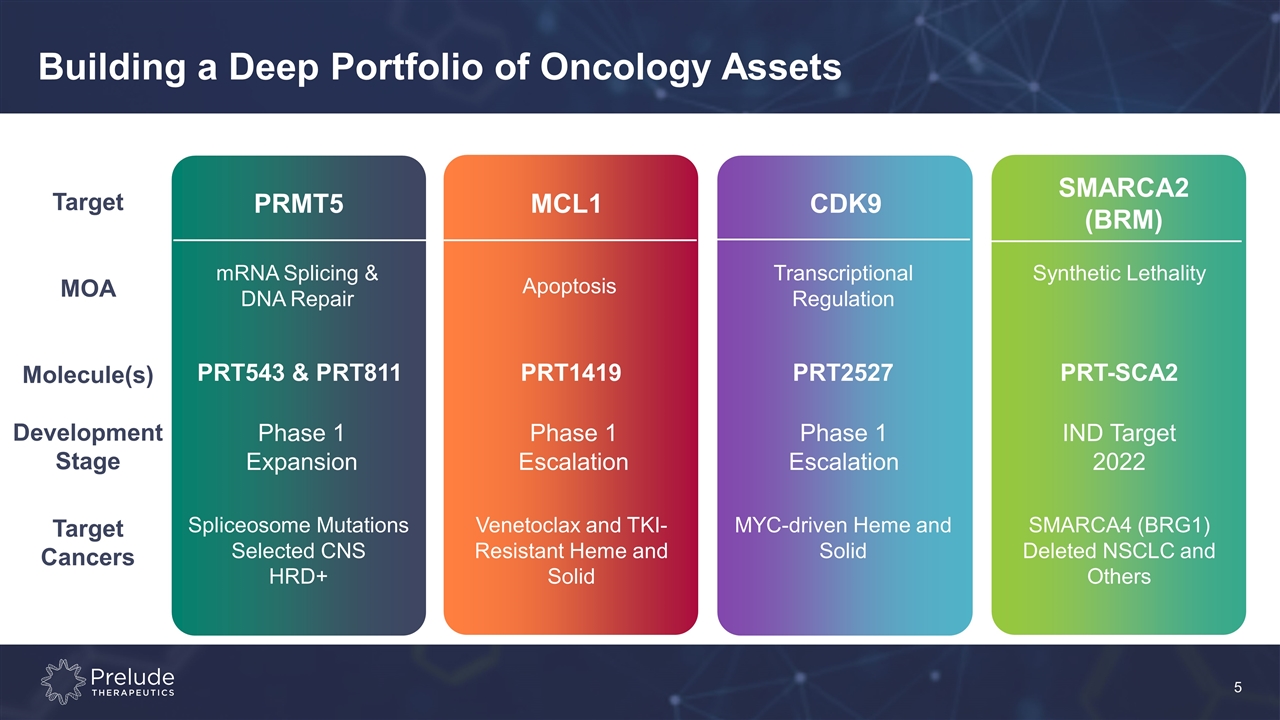
Target MOA Molecule(s) Development Stage Target Cancers Building a Deep Portfolio of Oncology Assets MCL1 CDK9 SMARCA2 (BRM) mRNA Splicing & DNA Repair PRT543 & PRT811 Phase 1 Expansion Spliceosome Mutations Selected CNS HRD+ Apoptosis PRT1419 Phase 1 Escalation Venetoclax and TKI- Resistant Heme and Solid Transcriptional Regulation PRT2527 Phase 1 Escalation MYC-driven Heme and Solid Synthetic Lethality PRT-SCA2 IND Target 2022 SMARCA4 (BRG1) Deleted NSCLC and Others PRMT5

Program Cancer Indications IND Enabling Phase 1 Escalation Phase 1 Expansion Phase 2/3 Upcoming Milestones PRT543 (PRMT5) ACC, HRD+, spliceosome mutations Data readouts 2H22 Selected myeloid malignancies (incl. MF and MDS) PRT811 (Brain Penetrant PRMT5) IDH+ high grade glioma, uveal melanoma Data readouts 2H22 PRT1419 (MCL1) Selected hematological malignancies and solid tumors Dose escalation data readout 2H22 PRT2527 (CDK9) Selected solid and hematological malignancies Dose escalation data YE 2022 PRT-SCA2 (SMARCA2) Multiple genomically selected cancers IND filing target 2022 PRT-K4 (Kinase) Solid tumors IND filing target 2022 Diversified Precision Oncology Pipeline

Complete expansion phases for both molecules Demonstrate PoC in one or more indications Advance PRMT5 program into Phase 2 Establish RP2D Demonstrate safety in combination with Venetoclax/Azacitidine Demonstrate PoC Complete dose escalation Establish safety, target engagement and RP2D File INDs Initiate Phase 1 studies PRT543 Identified RP2D Demonstrated favorable safety profile and preliminary clinical activity Multiple expansions initiated PRT811 Identified RP2D Best-in-class potential demonstrated; Expansion phase initiated Phase 1 dose escalation with oral formulation ongoing Phase 1 dose escalation with IV formulation initiated Successfully filed IND Phase 1 Initiated PRT-SCA2 - IND Enabling Studies Initiated; IND target in 2022 PRT-K4 - IND Enabling Studies Initiated; IND target in 2022 Preclinical ✓ ✓ ✓ ✓ 2021 Accomplishments and 2022 Goals 2021 2022 PRMT5 PRMT5 CDK9 MCL1

PRMT5 Program PRT543 & PRT811

PRMT5 Pathway Drives Oncogenesis and Resistance Transcription Factors PRMT5 Spliceosome Proteins Histones Symmetric Dimethylation PRMT5 catalyzes symmetric arginine dimethylation (sDMA) of protein substrates including histones, transcription factors, and spliceosome proteins Dimethylated substrates of PRMT5 control key oncogenic and resistance mechanisms PRMT5 inhibition is highly efficacious in models with mutations in DNA repair or mRNA splicing pathways in preclinical models PRMT5 inhibition can be leveraged to target genetically selected patient populations in the clinic DNA repair Drug Resistance Proliferation Tumor cell

PRT543 PRT811 Potential Best-In-Class PRMT5 Inhibitors Differentiated PRMT5 Inhibitors Highly selective and potent oral candidates PRT811 is highly differentiated in the class with high brain penetration potential Applicability in Both Solid Tumors and Heme Strong scientific rationale and robust preclinical activity across broad range of cancers Early clinical signals in multiple cancer types Optimized PK Profile High oral bioavailability and optimal half-life to maximize therapeutic window Differentiated safety and clinical activity profile Potential Rapid Path to Market Potential for accelerated approval pathway Opportunity in multiple cancer types

PRMT5: Phase 1 Overview Dose Escalation Unselected Patients Expansion Cohorts Selected Patients Solid Tumors GBM Myeloid Malignancies Recommended Expansion Doses PRT543 PRT811 Completed Ongoing PRT543 45 mg 5x/wk (solids) 35 mg 5x/wk (heme) PRT811 600 mg QD 1 2 3 4 5 6 7 Adenoid Cystic Carcinoma (N~40) Homologous Recombination Deficient (HRD+) Solid Tumors (N~40) Splicing mutated Solid and Myeloid Malignancies (N ~40) Uveal Melanoma High Grade Gliomas

PRT543 and PRT811 Demonstrate Desirable PK and PD Properties Dose-dependent Inhibition of Serum sDMA Dose-Dependent Increases in Cmax and AUC Data presented at 2021 AACR-NCI-EORTC Annual Meeting PRT543 PRT811

Durable CR in a patient with HRD+ ovarian cancer (35 mg 5x/wk; still on Rx) Stable disease and tumor regressions (<30%) in 5 patients including ACC and uveal melanoma Sustained hemoglobin and anemia improvements in multiple patients with myeloid malignancies Unselected patient population with advanced solid tumors and myeloid malignancies Safety Most common Grade ≥ 3 AEs were thrombocytopenia and anemia Thrombocytopenia is the only DLT Reversible upon dose modification Low incidence (<20%) at expansion doses Unselected patient population with advanced cancers including GBM Safety Grade ≥ 3 AEs were uncommon (occurring in 11% of patients) No DLTs up to 600 mg QD One patient with IDH1 mutated GBM experienced durable PR that evolved into CR (still on Rx) Two splicing mutated uveal melanoma patients demonstrated anti-tumor activity including a uPR and a 25% tumor regression PRT543 PRT811 PRMT5 - Phase 1 Dose Escalation: Safety and Clinical Activity* *Data presented at 2021 AACR-NCI-EORTC and ASH Annual Meetings Study Demographics & Safety Study Demographics & Safety Preliminary Clinical Activity Preliminary Clinical Activity

PRMT5 Phase 1: Key Takeaways and Next Steps Favorable Safety Profile Desirable PK & PD Profiles Preliminary Clinical Activity Next Steps PRT543 and PRT811 well tolerated Low incidence of dose-limiting toxicities at expansion doses Dose-dependent increase in exposure PRT811 demonstrated best-in-class profile with wide therapeutic window Objective responses in solid tumors Dose expansion ongoing Data readouts from multiple cohorts in 2022 Prioritize indications for advancement into P2 Favorable safety properties High levels of target inhibition Anti-tumor activity observed in relapsed/refractory patients with target biomarker profile IWG anemia benefit in myeloid malignancies

MCL1 Program PRT1419

MCL1: Targeting Cancer Cell Survival MCL1 is a member of family inhibitors of apoptosis (BCL2); often overexpressed in cancers BCL2 family is clinically validated – Venetoclax approved for lymphoid and myeloid malignacies MCL-1 is a bypass and resistance mechanism for venetoclax and multiple TKIs Challenging medicinal chemistry target that requires disruption of protein-protein interaction

PRT1419 Differentiated Clinical-Stage MCL1 Inhibitor Candidate MCL1 Inhibitor Potent and selective No cardiotoxicity signal in GLP-toxicology Studies Targeting Selected Heme and Solid Cancers Robust activity in preclinical models with once weekly dosing Potential combination strategy with Venetoclax and/or HMAs in Hematological malignancies Optimized PK Profile Maximizes Therapeutic Window Higher clearance built in to achieve desirable duration of target inhibition Optimal physicochemical properties Potential Rapid Path to Market Venetoclax-resistant cancers offer opportunity for accelerated approval

Status Dose escalation (monotherapy) ongoing Oral – Myeloid malignancies IV – Solid tumors Next Steps Combination cohorts Venetoclax and/or HMA Identify RP2D and initiate expansion phase Report dose escalation data (2H2022) Dose Escalation (ongoing) AML and High Risk MDS (Oral) Solid Tumors (IV) Recommended Expansion Dose Expansion Phase (Target 2H2022) Phase 1 Overview AML & High Risk MDS PRT1419 + Venetoclax or Aza (N~20) Potential Solid Tumors (N~20)

CDK9 Program PRT2527

CDK9: Targeting Cancer Through Transcriptional Regulation CDK9 phosphorylates RNA Pol II and regulates transcription Regulates expression of several immediate early genes driving oncogenesis and resistance (i.e. MYC, MYB, MCL1) Non-selective CDK9 inhibitors have demonstrated clinical activity in multiple tumor types but poor tolerability Lack of selectivity and potency vs other CDK9s is believed to contribute to low therapeutic window Super Enhancer RNA Pol II mRNA TSS MYC & MYB Target Genes

PRT2527 Potential Best-in-Class Selectivity and Potency CDK9 Inhibitor Most selective in the class vs CDK family and across the kinome Low nanomolar potency in blocking tumor cell proliferation Targeting Selected Heme and Solid Cancers Robust activity in preclinical models at well-tolerated doses Enhanced sensitivity in tumors that are MYC-dependent Provides patient selection strategy in clinic NSCLC PDX

Status Next Steps Phase 1 Overview Phase 1 Baysian Design (ongoing) MYC-dependent Solid Tumors and Hematologic Malignancies MYC-dependent solid tumors Sarcoma Hematologic malignancies PoC Phase 2 (Target 1H 2023) Complete Phase 1 Select RP2D Initiate Phase 2 Dose Escalation Solid tumors Hematological malignancies

SMARCA2 (BRM) Program

The chromatin remodeling (SWI/SNF) complex is frequently mutated in cancer making it a potential therapeutic target Activity of the SWI/SNF complex requires either SMARCA4 (BRG1) or SMARCA2 (BRM) Loss of SMARCA4 (BRG1) through mutation leads to dependency on SMARCA2 (BRM) Subsets of solid tumors express SMARCA4 (BRG1) mutations Selectively inhibiting SMARCA2 (BRM) offers an attractive approach to target SMARCA4 (BRG1) mutant tumors Targeting SMARCA2 (BRM): Leveraging Synthetic Lethality

Achieving SMARCA2 Selectivity Through Degrader Approach SMARCA2 selectivity over highly homologous SMARCA4 isoform has been a challenging medicinal chemistry problem with traditional small molecule approaches Targeted Protein Degradation (TPD) of SMARCA2 selectively over SMARCA4 is possible through differences in ternary complexes Prelude scientists identified the molecular basis for achieving high degree of selectivity for SMARCA2 over SMARCA4 Lead molecules from multiple chemical scaffolds with sub-nanomolar potency and selectivity have been discovered Small Molecule Degrader E3 Ligase Target Protein Proteasome Ubiquitinated Target Protein Mullard A. Nat Rev Drug Discov. 2019

PRT-SCA2: Potent and Selective SMARCA2 Degrader with in Vivo Activity Prelude SMARCA2 Degraders Replicate Genetic Synthetic Lethality Highly Selective for SMARCA2 Degradation SMARCA2 Actin Veh 8h 24h SMARCA4 Hours after dosing In vitro In vivo HT1080 SM4 KO DMSO 0.1 1.0 10 100 1000 PRT007(nM): Calu-6 NCI-H520 NCI-H1693 NCI-H838 SM4 WT HT1080 WT SM4-del Robust Tumor Growth Inhibition of SMARCA4 mutated but not WT Xenograft SMARCA4 mutant SMARCA4 WT

Status Next Steps Candidate molecule(s) identified IND enabling studies initiated File IND 2022 Initiate Phase 1 escalation study in SMARCA4 mutant cancers SMARCA4 Mutated NSCLC and other tumor types Phase 1 Dose Escalation Target 2H 2022 SMARCA2 Degrader Program Overview IND Filing IND Enabling Studies

Corporate Highlights

Our Investment Thesis Internal discovery engine Differentiated R&D approach Strong execution and commitment to discovery Focused clinical development in underserved cancers Strong financial position: ~$320M cash and marketable securities at 9/30/21 PMRT5 MCL1 CDK9 SMARCA2 (BRM) Data Rich 2022

Thank You
